
Flexible Manufacturing in Pharma with CDMOs
Shots:
-
Pharmaceutical manufacturing involves complex procedures like blending, compression, filtration, heating, encapsulation, shearing, tableting, granulation, coating, and drying. Traditionally, for all these processes, pharma companies had designated factory floors to carry out the process smoothly
-
The flexible manufacturing system (FMS) revolutionized the course of manufacturing, especially in the pharma industry. Contract Development Manufacturing Organizations' (CDMOs) popularity grew exponentially with the advent of COVID-19 and disrupted manufacturing in the pharma industry forever
-
The article navigates the aspects of flexible manufacturing in the pharma industry and the role of CDMOs in expediting the overall drug development process
“We cannot solve a problem with the same thinking we used when we created them,” a famous quote from Einstein invokes a sense of resilience and flexibility that we as an individual and a business need to cope with the changing dynamics of the market.
The Pharma Industry invests a fortune to facilitate the growing and never-ending needs of the healthcare sector. Some investments turn out to be in the black, while others remain at loose ends. Encapsulated with uncertainties in moving ahead in clinical phases, regulatory landscapes, and pressure from investors and stakeholders, the pharma companies make prudent decisions before any merger and acquisition. Pharma companies often devise elaborate strategies to go through the infrastructural layout before making any investment, and in doing so, the most sought-after facility that companies look for is the Flexible Manufacturing System (FMS).
The article navigates the aspects of FMS, its advantages, challenges, the evolving role of CDMOs in pharmaceutical manufacturing, leading companies, and an insightful conclusion.
What is Flexible Manufacturing System (FMS)?
The flexible manufacturing system uses a modular setup for production, using a customizable factory floor. With FMS, pharma companies can open doors to unlimited opportunities, allowing companies to explore multiple aspects of drug development conveniently. Infrastructure plays a crucial role in developing FMS for any industry. FMS facilities are predominant in CDMOs, allowing them to handle multiple stages of production and packaging.
The ongoing COVID-19 pandemic had once halted the entire secondary industry and disrupted the manufacturing process. CDMOs remained unaffected due to the use of a flexible manufacturing system. With constant innovation in the industry, traditional manufacturing floors offer limited scope for integration. In such cases, modular FMS factory floors come in handy with their customizable options for enhancing the production level.
Moreover, traditional floors are at higher risk of cross-contamination as compared to FMS facilities while shifting from one product to another. FMS facilities are instrumental in revolutionizing the ways of production by being more compatible and efficient as compared to conventional manufacturing systems.
Advantages of Flexible Manufacturing System
A flexible manufacturing system offers a substantial advantage in the pharmaceutical industry. Some of the key benefits that come with FMS are:
-
Space savvy
-
Allows room for constant production
-
Ready to produce factory floor in case of emergencies
-
Shortens the development phase
-
Efficient testing
-
Quick changeovers
-
Lower risk of contamination
Challenges in FMS Facilities
Leveraging FMS in the life science industry can be challenging, especially for small companies. Let’s take a quick look on the major challenges of FMS
-
Requires extensive planning
-
Modular setups may incur hefty expenses
-
Requires highly trained professionals
-
Requires the use of advanced equipment
FMS in the Pharma Industry
With plenty of untapped opportunities waiting to be explored, pharma companies are toiling day and night to infuse new-age technologies and practices prevalent in the industry. By infusing flexible manufacturing systems, pharma companies open the door to new processes and products. With rigid manufacturing floors, pharmaceutical companies are left with no other options than to outsource drug development through contract development and manufacturing organizations (CDMOs). Flexible production lines help identify cross-over steps and allow pharma companies to leverage the same piece of equipment in similar processes. Flexible manufacturing lines help pharmaceutical companies prevent downtime by offering highly-paced testing and manufacturing facilities.
Outsourcing Production via. CDMOs
Around 60% of pharmaceutical manufacturing is outsourced via. CDMOs. The reason for this can be attributed to the fact that >50% of strategic partnerships fail after a few years. The inability to sustain the terms of alliances, especially in cross-border partnerships, can be attributed to various detrimental factors.
In such scenarios, CDMOs, with their integrated procurement and supply chain management, offer significant advantages for pharmaceutical companies by emerging as strategic manufacturing partners. Some of the key benefits of outsourcing the production via. CDMOs are:
-
Scalability: By outsourcing product development through CDMOs, companies eliminate the risks involved with in-house production. CDMOs provide cost-effective solutions when companies look for the transition from drug discovery to production
-
Cutting-edge technologies: On a trail to high-end and advanced equipment, CDMOs offer safe abodes to pharma companies. With the incessant innovation in the industry, availing of advanced equipment may be challenging. To meet the needs of ever-changing technological advances, CDMOs constantly upgrade their types of machinery
-
Easy Integration into different processes: Rigid factory floors have minimal chances of introducing change while switching processes. In such cases, CDMOs come in handy with their modular setup.
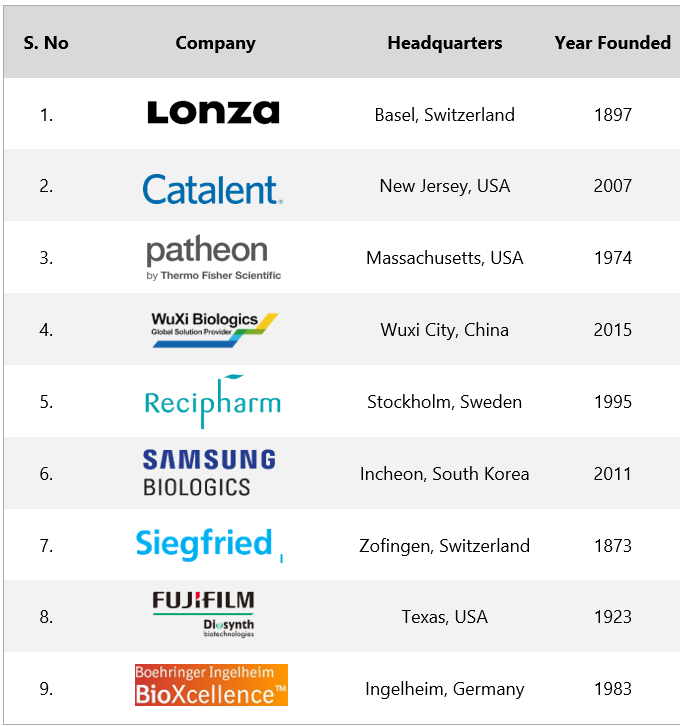
Leading CDMOs in the Market
-
Lonza: Founded in 1897 and headquartered in Basel Switzerland, Lonza is a multinational manufacturing company for the pharma, biotech, and nutrition markets. The company focuses on biologics, small molecules, cell & gene therapy, and capsules & health ingredients. In 2022, Lonza generated a whopping revenue of $6.7B
-
Catalent: A multinational corporation headquartered in New Jersey, USA, Catalent delivers, develops, and manufactures drugs, biologics, gene therapies, and consumer health products. Catalent was founded in 2007 and gradually rose to prominence and in 2019, Catalent went public. In 2022, Catalent generated $4.82B in revenue.
-
Patheon (ThermoFisher): Headquartered in Massachusetts, Patheon is a renowned contract manufacturer that provides end-to-end services on different phases of drug development, APIs, logistics, and more.
-
WuXi Biologics: Wuxi Biologics is a global research CDMO that provides an integrated technology platform for biologics. Founded in 2015, Wuxi Biologics leverages the use of new-age technologies to provide advanced solutions to its clients to discover cost-effective biologics, development, and manufacturing solutions.
-
Recipharm: Recipharm, was founded in 1995, and now it’s a leading name in the CDMO industry, helping the pharmaceutical fraternity by offering contract development solutions. Founded in 1995 and headquartered in Stockholm Sweden, in 2022, the revenue of Recipharm was $1.3B
-
Samsung Biologics: Samsung Biologics discovers, develops, and manufactures biologics for biopharma companies. Headquartered in South Korea, Samsung Biologics was founded in 2011 and is now a notable name among the CDMO fraternity. In 2022, Samsung Biologics revenue surged to $1.93B
-
Siegfreid: Siegfried is a renowned name in drug development, manufacturing, and packaging. Founded in 1873, Siegfried leverages advanced technologies to help biopharma companies develop products, starting from molecule selection to market launch and commercial supply.
-
Fujifilm Diosynth: A leading CDMO that develops and manufactures biologics, vaccines, and advanced therapies, Fujifilm Diosynth was founded in 1923 and is headquartered in Texas, USA. Fujifilm is accredited to offer complete solutions in pharmaceutical manufacturing.
-
Boehringer Ingelheim BioXcellence: Boehringer Ingelheim BioXcellence provides global contract development and manufacturing services to pharma and biotech companies. Boehringer Ingelheim's first biologic Berofor in 1983 laid the foundation stone of BioXcellence.
What Does the Future Hold for CDMOs?
In 2022, CDMOs market size was valued at $94.17B and is anticipated to cross the threshold of $172.02B by 2032 with a CAGR of 6.21%. The contract outsourcing industry has proven to be a beacon of hope for pharma companies. CDMOs provide a robust network to ease the advanced production of pharmaceutical products. Being cost-effective, leveraging CDMOs becomes the only way out to avert the crisis emanating from high market demands and rigid factory floors. CDMOs have prolifically adopted the single-use system in which equipment like disposable bioreactors and others are used instead of the stainless-steel equipment that you see in rigid manufacturing systems. Single-use equipment is mostly made of plastic and exposed to gamma irradiation to sterilize them, therefore avoiding the risks of contamination during product development.
Conclusions & Perspectives
So far, we have navigated the multiple aspects of flexible manufacturing systems and the growing popularity of CDMOs in the life science industry. Biopharma companies can effectively dispense development and manufacturing activities to CDMOs and in doing so, save a bulk load of resources that can be allocated to strengthen R&D and run market campaigns.
Take advantage of Octavus Consulting’s life science solutions! PharmaShots’ parent company, Octavus, specializes in catering to the growing needs of pharmaceutical, biopharma, biotech, MedTech, life science, and animal health industries with their real-time and real-world competitive and business intelligence services. For more information, reach out to us at connect@pharmashots.com or bd@octavusconsulting.com
References:
Related Post: Medication-Assisted Recovery: Enhancing Outcomes In Dual Diagnosis Scenarios
Tags

Saurabh is a Senior Content Writer at PharmaShots. He is a voracious reader and follows the recent trends and innovations of life science companies diligently. His work at PharmaShots involves writing articles, editing content, and proofreading drafts. He has a knack for writing content that covers the Biotech, MedTech, Pharmaceutical, and Healthcare sectors.













